Abstract
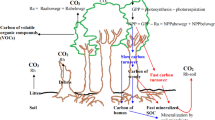
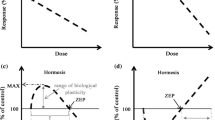
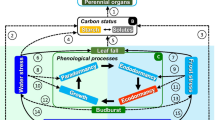
Woody plants contribute to the stability and productivity of terrestrial ecosystems and are significantly affected by climate change. According to the concept of environmental hormesis, any environmental stressors can cause hormesis, that is, stimulation in low doses and inhibition in high doses. Numerous studies have demonstrated plant hormesis under low doses of various abiotic stressors. However, the hormetic responses of woody plants to abiotic stressors from climate change are insufficiently studied. This review analyses data on the stimulating effects of low doses of climate stressors in experiments and in real ecosystems. Numerous laboratory and field experiments show that single and combined exposure to various climate stressors (temperature, humidity, and elevated carbon dioxide concentrations) can cause hormesis in various species and functional types of woody plants, which can be accompanied by hormetic trade-offs and preconditioning. In addition, there is evidence of climate hormesis in woody plants in ecosystem conditions. Field experiments in various ecosystems show that elevated temperatures and/or precipitation or elevated carbon dioxide concentrations causing hormesis in dominant tree species can stimulate ecosystem productivity. Moreover, climate hormesis of the growth and reproduction of dominant forest tree species contributes to the spread of forests, that is, climate-driven ecological succession. The main commonalities of climate hormesis in woody species include: (1) Low-dose climate stressors cause hormesis in woody plants when strong (limiting) stressors do not affect plants or these limiting stressors are mitigated by climate change. (2) Hormesis can occur with the direct impact of climatic stressors on trees and with the indirect impact of these stressors on plants through other parts of the ecosystem. (3) Climate stressor interactions (e.g., synergism, antagonism) can affect hormesis. (4) Hormesis may disappear due to tree acclimatization with consequent changes in the range of tolerances to climate factors. This review highlights the need for targeted studies of climate hormesis in woody species and its role in the adaptation of forest ecosystems to climate change.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Agathokleous E (2018) Environmental hormesis, a fundamental non-monotonic biological phenomenon with implications in ecotoxicology and environmental safety. Ecotox Environ Safe 148:1042–1053. https://doi.org/10.1016/j.ecoenv.2017.12.003
Agathokleous E, Calabrese EJ (2020) A global environmental health perspective and optimisation of stress. Sci Total Environ 704:135263. https://doi.org/10.1016/j.scitotenv.2019.135263
Agathokleous E, Calabrese EJ (2022) Hormesis: a general biological principle. Chem Res Toxicol 35(4):547–549. https://doi.org/10.1021/acs.chemrestox.2c00032
Agathokleous E, Kitao M, Harayama H, Calabrese EJ (2019) Temperature-induced hormesis in plants. J for Res 30:13–20. https://doi.org/10.1007/s11676-018-0790-7
Agathokleous E, Kitao M, Calabrese EJ (2020) Hormesis: highly generalizable and beyond laboratory. Trends Plant Sci 25(11):1076–1086. https://doi.org/10.1016/j.tplants.2020.05.006
Agathokleous E, Iavicoli I, Barceló D, Calabrese EJ (2021) Micro/nanoplastics effects on organisms: a review focusing on ‘dose.’ J Hazard Mater 417:126084. https://doi.org/10.1016/j.jhazmat.2021.126084
Agathokleous E, Barceló D, Rinklebe J, Sonne C, Calabrese EJ, Koike T (2022) Hormesis induced by silver iodide, hydrocarbons, microplastics, pesticides, and pharmaceuticals: implications for agroforestry ecosystems health. Sci Total Environ 820:153116. https://doi.org/10.1016/j.scitotenv.2022a.153116
Agathokleous E, Guedes RNC, Calabrese EJ, Fotopoulos V, Azevedo RA (2022) Transgenerational hormesis: what do parents sacrifice for their offspring? Curr Opin Environ Sci Health 29:100380. https://doi.org/10.1016/j.coesh.2022b.100380
Agathokleous E, Peñuelas J, Azevedo RA, Rillig MC, Sun H, Calabrese EJ (2022c) Low levels of contaminants stimulate harmful algal organisms and enrich their toxins. Environ Sci Technol 56(17):11991–12002. https://doi.org/10.1021/acs.est.2c02763
Agathokleous E, Wang Q, Iavicoli I, Calabrese EJ (2022d) The relevance of hormesis at higher levels of biological organization: hormesis in microorganisms. Curr Opin Toxicol 29:1–9. https://doi.org/10.1016/j.cotox.2021.11.001
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? a meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165(2):351–371. https://doi.org/10.1111/j.1469-8137.2004.01224.x
Allen LH, Vu JCV (2009) Carbon dioxide and high temperature effects on growth of young orange trees in a humid, subtropical environment. Agr Forest Meteorol 149:820–830
Apple ME, Olszyk DM, Ormrod DP, Lewis A, Southworth D, Tingey DT (2000) Morphology and stomatal function of Douglas fir needles exposed to climate chance: elevated CO2 and temperature. Inter J Plant Sci 161:127–132
Barker DH, Loveys BR, Egerton JJG, Gorton H, Williams WE, Ball MC (2005) CO2 enrichment predisposes foliage of a eucalypt to freezing injury and reduces spring growth. Plant Cell Environ 28(12):1506–1515. https://doi.org/10.1111/j.1365-3040.2005.01387.x
Belz RG, Carbonari CA, Duke SO (2022) The potential influence of hormesis on evolution of resistance to herbicides. Curr Opin Environ Sci Health 27:100360. https://doi.org/10.1016/j.coesh.2022.100360
Bunn AG, Goetz SJ, Kimball JS, Zhang K (2007) Northern high-latitude ecosystems respond to climate change. EOS Trans Am Geophys Union 88:333–335. https://doi.org/10.1029/2007EO340001
Buriánek V, Novotný R, Hellebrandová K, Šrámek V (2013) Ground vegetation as an important factor in the biodiversity of forest ecosystems and its evaluation in regard to nitrogen deposition. J For Sci 59:238–252
Caignard T, Kremer A, Firmat C, Nicolas M, Venner S, Delzon S (2017) Increasing spring temperatures favor oak seed production in temperate areas. Sci Rep 7:8555. https://doi.org/10.1038/s41598-017-09172-7
Calabrese EJ (2008) Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem 27(7):1451–1474. https://doi.org/10.1897/07-541
Callaway RM, DeLucia EH, Thomas EM, Schlesinger WH (1994) Compensatory responses of CO2 exchange and biomass allocation and their effects on the relative growth rate of ponderosa pine in different CO2 and temperature regimes. Oecologia 98:159–166
Centritto M, Lee HSJ, Jarvis PG (2008) Increased growth in elevated [CO2]: an early, short-term response? Global Change Biol 5(6):623–633. https://doi.org/10.1046/j.1365-2486.1999.00263.x
de Dato G, Pellizzaro G, Cesaraccio C, Sirca C, De Angelis P, Duce P, Spano D, Scaracia MG (2008) Effects of warmer and drier climate conditions on plant composition and biomass production in a Mediterranean shrubland community. iForest 1:39–48
DeLucia EH, Callaway RM, Schlesinger WH (1994) Offsetting changes in biomass allocation and photosynthesis in ponderosa pine (Pinus ponderosa) in response to climate change. Tree Physiol 14(7–9):669–677. https://doi.org/10.1093/treephys/14.7-8-9.669
Devi NM, Kukarskih VV, Galimova AA, Mazepa VS, Grigoriev AA (2020) Climate change evidence in tree growth and stand productivity at the upper treeline ecotone in the Polar Ural mountains. For Ecosyst 7:7. https://doi.org/10.1186/s40663-020-0216-9
Dormann CF, Woodin SJ (2002) Climate change in the arctic: using plant functional types in a meta-analysis of field experiments. Funct Ecol 16:4–17. https://doi.org/10.1046/j.0269-8463.2001.00596.x
Erofeeva EA (2021) Plant hormesis and Shelford’s tolerance law curve. J for Res 32:1789–1802. https://doi.org/10.1007/s11676-021-01312-0
Erofeeva EA (2022) Environmental hormesis of non-specific and specific adaptive mechanisms in plants. Sci Total Environ 804:150059. https://doi.org/10.1016/j.scitotenv.2021.150059
Erofeeva EA (2022) Hormesis in plants: its common occurrence across stresses. Curr Opin Toxicol 30:100333. https://doi.org/10.1016/j.cotox.2022b.02.006
Erofeeva EA (2022) Environmental hormesis: from cell to ecosystem. Curr Opin Environ Sci Health 29:100378. https://doi.org/10.1016/j.coesh.2022c.100378
Fatholahi M, Fallah A, Hojjati SM, Kalbi S (2017) Estimation of aboveground tree carbon stock using SPOT-HRG data (a case study: Darabkola forests). J for Res 28:1177–1184. https://doi.org/10.1007/s11676-017-0396-5
Friedrichs DA, Büntgen U, Frank DC, Esper J, Neuwirth B, Löffler J (2009) Complex climate controls on twentieth century oak growth in Central-West Germany. Tree Physiol 29(1):39–51
Gedalof Z, Berg AA (2010) Tree ring evidence for limited direct CO2 fertilization of forests over the Twentieth century. Global Biogeochem Cycles 24(3):GB3027. https://doi.org/10.1029/2009GB003699
Gregg JW, Jones CG, Dawson TE (2003) Urbanization effects on tree growth in the vicinity of New York city. Nature 424:183–187. https://doi.org/10.1038/nature01728
Heilman KA, Trouet VM, Belmecheri S, Pederson N, Berke MA, McLachlan JS (2021) Increased water use efficiency leads to decreased precipitation sensitivity of tree growth, but is offset by high temperatures. Oecologia 197:1095–1110. https://doi.org/10.1007/s00442-021-04892-0
Huang J, Bergeron Y, Denneler B, Berninger F, Tardif J (2007) Response of forest trees to increased atmospheric CO2. Crit Rev Plant Sci 26(5–6):265–283. https://doi.org/10.1080/07352680701626978
IPCC (2021) Climate change 2021: The physical science Basis. In: Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press, New York, pp. 113–119
IPCC (2022) Summary for policymakers Pӧrtner HO, Roberts DC, Poloczanska ES, Mintenbeck K, Tignor M, Alegría A, Craig M, Langsdorf S, Lӧschke S, Mӧller V, Okem A (eds.). In: Climate change 2022: Impacts, adaptation, and vulnerability. contribution of working group II to the sixth assessment report of the intergovernmental panel on climate Change Pӧrtner HO, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Lӧschke S, Mӧller V, Okem A, Rama B (eds.). Cambridge University Press
Kauppi PE, Posch M, Pirinen P (2014) Large impacts of climatic warming on growth of boreal forests since 1960. PLoS One 9(11):e111340. https://doi.org/10.1371/journal.pone.0111340
Kijowska-Oberc J, Staszak AM, Kamiński J, Ratajczak E (2020) Adaptation of forest trees to rapidly changing climate. Forests 11:123. https://doi.org/10.3390/f11020123
Kuokkanen K, Niemela P, Matala J, Julkunen-Tiitto R, Heinonen J, Rousi M, Henttonen H, Tahvanainen J, Kellomaki S (2004) The effects of elevated CO2 and temperature on the resistance of winter-dormant birch seedlings (Betula pendula) to hares and voles. Global Change Biol 10:1504–1512
Li Y, Xu Y, Li Y, Wu T, Zhou G, Liu S, Meng Y, Wang J, Ling L, Liu J (2020) Warming effects on morphological and physiological performances of four subtropical montane tree species. Ann For Sci 77:2. https://doi.org/10.1007/s13595-019-0910-3
Lovelock CE, Virgo A, Popp M, Winter K (1999) Effects of elevated CO2 concentrations on photosynthesis, growth and reproduction of branches of the tropical canopy tree species, Luehea seemannii Tr. and Planch. Plant Cell Environ 22(1):49–59. https://doi.org/10.1046/j.1365-3040.1999.00370.x
Maury S, Le Gac AL, Lafon-Placette C, Sow MD, Fichot R, Delaunay A, Le Jan I, Lelu-Walter MA, Segura V, Rogier O, Trontin JF, Plomion C, Le Provost G, Ehrenmann F, Salse J, Ambroise C, Gribkova S, Mirouze M, Grunau C, Chaparro C, Strauss SH, Conde D, Allona I, Tost J (2018) Epigenetics in trees: a source of plasticity and adaptation in the context of climate change. In: Proceeding of the 5th international conference of the IUFRO unit on “Clonal Trees in the bioeconomy age: opportunities and challenges” September 10–15, Coimbra, Portugal P. pp 110–115
McLean S, Richards SM, Cover SL (2009) Papyriferic acid, an antifeedant triterpene from birch trees, inhibits succinate dehydrogenase from liver mitochondria. J Chem Ecol 35:1252. https://doi.org/10.1007/s10886-009-9702-9
Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, Bowles FP, Catricala C, Magill A, Ahrens T, Morrisseau S (2002) Soil warming and carbon-cycle feedbacks to the climate system. Science 298(5601):2173–2176. https://doi.org/10.1126/science.1074153
Motai A, Terada Y, Kobayashi A, Saito D, Shimada H, Yamaguchi M, Izuta T (2017) Combined effects of irrigation amount and nitrogen load on growth and needle biochemical traits of Cryptomeria japonica seedlings. Trees 31:1317–1333
Njana MA, Mbilinyi B, Eliakimu Z (2021) The role of forests in the mitigation of global climate change: emprical evidence from Tanzania. Environ Chall 4:100170. https://doi.org/10.1016/j.envc.2021.100170
Odum EP, Barrett GW (2004) Fundamentals of ecology. Brooks Cole, Belmont
Overdieck D, Ziche D, Bottcher-Jungclaus K (2007) Temperature responses of growth and wood anatomy in European beech saplings grown in different carbon dioxide concentrations. Tree Physiol 27:261–268
Park SW, Kim JS, Kug JS (2020) The intensification of arctic warming as a result of CO2 physiological forcing. Nat Commun 11:2098. https://doi.org/10.1038/s41467-020-15924-3
Peñuelas J, Prieto P, Beier C, Cesaraccio C, de Angelis P, de Dato G, Emmett BA, Estiarte M, Garadnai J, Gorissen A, Láng EK, Kröel-Dulay G, Llorens L, Pellizzaro G, Riis-Nielsen T, Schmidt IK, Sirca C, Sowerby A, Spano D, Tietema D (2007) Response of plant species richness and primary productivity in shrublands along a north–south gradient in Europe to 7 years of experimental warming and drought: reductions in primary productivity in the heat and drought year of 2003. Global Change Biol 13:2563–2581. https://doi.org/10.1111/j.1365-2486.2007.01464.x
Piggott JJ, Townsend CR, Matthaei CD (2015) Reconceptualizing synergism and antagonism among multiple stressors. Ecol Evol 5(7):1538–1547. https://doi.org/10.1002/ece3.1465
Pretzsch H, Biber P, Schütze G, Uhl E, Rötzer T (2014) Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat Commun 5:4967. https://doi.org/10.1038/ncomms5967
Pretzsch H, Biber P, Uhl E, Dahlhausen J, Schütze G, Perkins D, Rötzer T, Caldentey J, Koike T, Con TV, Chavanne A, Toit BD, Foster K, Lefer B (2017) Climate change accelerates growth of Urban trees in metropolises worldwide. Sci Rep 7(1):15403. https://doi.org/10.1038/s41598-017-14831-w
Reuveni J, Bugbee B (1997) Very high CO2 reduces photosynthesis, dark respiration and yield in wheat. Ann Bot 80(4):539–546. https://doi.org/10.1006/anbo.1997.0489
Reyer CPO, Bathgate S, Blennow K, Borges JG, Bugmann H, Delzon S, Faias SP, Garcia-Gonzalo J, Gardiner B, Gonzalez-Olabarria JR, Gracia C, Hernández JG, Kellomäki S, Kramer K, Lexer MJ, Lindner M, van der Maaten E, Maroschek M, Muys B, Nicoll B, Palahi M, Palma JH, Paulo JA, Peltola H, Pukkala T, Rammer W, Ray D, Sabaté S, Schelhaas MJ, Seidl R, Temperli C, Tomé M, Yousefpour R, Zimmermann NE, Hanewinkel M (2017) Are forest disturbances amplifying or canceling out climate change-induced productivity changes in European forests? Environ Res Lett 12(3):034027. https://doi.org/10.1088/1748-9326/aa5ef1
Rustad LE, Campbell JL, Marion GM, Norby R, Mitchell M, Hartley A, Cornelissen J, Gurevitch J (2001) GCTE-NEWS a meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562. https://doi.org/10.1007/s004420000544
Schneider C, Neuwirth B, Schneider S, Balanzategui D, Elsholz S, Fenner D, Meier F, Heinrich I (2022) Using the dendro-climatological signal of Urban trees as a measure of urbanization and urban heat Island. Urban Ecosyst 25:849–865. https://doi.org/10.1007/s11252-021-01196-2
Searle SY, Turnbull MH, Boelman NT, Schuster WSF, Dan Y, Griffin KL (2012) Urban environment of New York city promotes growth in northern red oak seedlings. Tree Physiol 32(4):389–400. https://doi.org/10.1093/treephys/tps027
Silva LCR, Sun G, Zhu-Barker X, Liang Q, Wu N, Horwath WR (2016) Tree growth acceleration and expansion of alpine forests: the synergistic effect of atmospheric and edaphic change. Sci Adv 2:e1501302
Smith SD, Huxman TE, Zitzer SF, Charlet TN, Housman DC, Coleman JS, Fenstermaker LK, Seemann JR, Nowak RS (2000) Elevated CO2 increases productivity and invasive species success in an arid ecosystem. Nature 408(6808):79–82. https://doi.org/10.1038/35040544
Smith WK, Reed SC, Cleveland CC, Ballantyne AP, Anderegg WR, Wieder WR, Liu YY, Running SW (2015) Large divergence of satellite and earth system model estimates of global terrestrial CO2 fertilization. Nat Clim Change 6(3):306–310
Su Y, Renz M, Cui B, Sun X, Ouyang Z, Wang X (2021) Leaf morphological and nutrient traits of common woody plants change along the urban–rural gradient in Beijing China. Front Plant Sci 12:682274. https://doi.org/10.3389/fpls.2021.682274
Trontin JF, Raschke J, Rupps A (2021) Tree ‘memory’: new insights on temperature-induced priming effects during early embryogenesis. Tree Physiol 41(6):906–911. https://doi.org/10.1093/treephys/tpaa150
Ueyama M, Ichii K, Kobayashi H, Kumagai T, Beringer J, Merbold L, Euskirchen ES, Hirano T, Marchesini LB, Baldocchi D, Saitoh TM, Mizoguchi Y, Ono K, Kim J, Varlagin A, Kang M, Shimizu T, Kosugi Y, Bret-Harte MS, Machimura T, Matsuura Y, Ohta T, Takagi K, Takanashi S, Yasuda Y (2020) Inferring CO2 fertilization effect based on global monitoring land-atmosphere exchange with a theoretical model. Environ Res Lett 15:084009
Usami T, Lee J, Oikawa T (2008) Interactive effects of increased temperature and CO2 on the growth of Quercus myrsinaefolia saplings. Plant Cell Environ 24(10):1007–1019. https://doi.org/10.1046/j.1365-3040.2001.00753.x
Vinayak B, Lee HS, Gedam S, Latha R (2022) Impacts of future urbanization on urban microclimate and thermal comfort over the Mumbai metropolitan region India. Sustain Cities Soc 79:103703. https://doi.org/10.1016/j.scs.2022.103703
Wang S, Huang B, Fan D, Agathokleous E, Guo Y, Zhu Y, Han J (2021) Hormetic responses of soil microbiota to exogenous Cd: a step toward linking community-level hormesis to ecological risk assessment. J Hazard Mater 416:125760. https://doi.org/10.1016/j.jhazmat.2021.125760
Way DA, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30(6):669–688. https://doi.org/10.1093/treephys/tpq015
Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA (2011) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Global Change Biol 17(2):927–942. https://doi.org/10.1111/j.1365-2486.2010.02302.x
Xiao Y, Wang M, Song Y (2022) Abiotic and biotic stress cascades in the era of climate change pose a challenge to genetic improvements in plants. Forests 13:780. https://doi.org/10.3390/f13050780
Yakovlev IA, Carneros E, Lee Y, Olsen JE, Fossdal CG (2016) Transcriptional profiling of epigenetic regulators in somatic embryos during temperature induced formation of an epigenetic memory in Norway spruce. Planta 243:1237–1249. https://doi.org/10.1007/s00425-016-2484-8
Yang J, Medlyn BE, De Kauwe MG, Duursma RA, Jiang MK, Kumarathunge D, Crous KY, Gimeno TE, Wujeska-Klause A, Ellsworth DS (2020) Low sensitivity of gross primary production to elevated CO2 in a mature eucalypt woodland. Biogeosciences 17(2):265–279. https://doi.org/10.5194/bg-17-265-2020
Yuan Y, Ge L, Yang H, Ren W (2018) A meta-analysis of experimental warming effects on woody plant growth and photosynthesis in forests. J For Res 29(3):727–733. https://doi.org/10.1007/s11676-017-0499-z
Zhao H, Li Y, Zhang X, Korpelainen H, Li C (2012) Sex-related and stage-dependent source-to-sink transition in Populus cathayana grown at elevated CO2 and elevated temperature. Tree Physiol 32(11):1325–1338. https://doi.org/10.1093/treephys/tps074
Zheng Y, Li F, Hao L, Yu J, Guo L, Zhou H, Ma C, Zhang X, Xu M (2019) Elevated CO2 concentration induces photosynthetic down-regulation with changes in leaf structure, non-structural carbohydrates and nitrogen content of soybean. BMC Plant Biol 19:255. https://doi.org/10.1186/s12870-019-1788-9
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The online version is available at http://www.springerlink.com.
Guest editor: Yanbo Hu.
Corresponding editor: Yanbo Hu.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erofeeva, E.A. Hormetic effects of abiotic environmental stressors in woody plants in the context of climate change. J. For. Res. 34, 7–19 (2023). https://doi.org/10.1007/s11676-022-01591-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-022-01591-1